Samples have been monitored each day by counting the entire amount of discrete colonies on Each individual plate and checking the turbidity of TSB tubes. Constructive and unfavorable Manage agar plates and TSB tubes ended up ready and incubated simultaneously.
All workers qualified to work in the area, such as maintenance personnel, need to be included in APS.
This guidance is meant to help you producers satisfy the requirements within the Agency's present very good manufacturing practice (CGMP) polices (2l CFR parts 210 and 211) when manufacturing sterile drug and biological merchandise employing aseptic processing.
The authors been given no economic help with the analysis, authorship, and/or publication of this short article.
An Ishikawa diagram demonstrating lead to-and-result backlinks to a specific failure is a useful tool which might be applied to research and recognize the foundation explanation for a media fill failure (see Figure 2).
Leakage from filling needle in the course of filling Procedure that ends in the repeated intervention of filling needle adjustment and cleansing of the spilled product or service less than Grade A.
Enough filled media containers should be sampled from the beginning and website stop of every APS to carry out progress promotion of all organisms on each established.
The fill volume of media needs to be enough to moist the entire area including the closures and to permit simple inspection. A volume of at least increased than 50 % of the whole container quantity is suggested.
Sterile drug output has often been a tough job to complete inside the pharmaceutical industry. There are several prerequisites to fulfill: to guarantee item quality, to safeguard the operator when potent compounds are current, to obtain higher amounts of performance,.
Simulate all routine and doable non-program interventions throughout media fill as per the described method.
Incubate filled units in skilled incubators monitored by skilled and calibrated temperature checking units.
These involved no prior disinfection of surfaces, gear or gloves, and intentional finger dab around the septum and to the luer-lok stoppers of vials. Incubation and day-to-day observation were executed in the same way to the procedures made use of all through operators assessment.
Editor’s Preference articles are according check here to recommendations from the scientific editors of MDPI journals from around the globe.
Sterilization is undoubtedly an absolute phrase, and microbiologists try to realize this point out in A lot from the preparation do the job which they do by way of a variety of processes ordinarily involving warmth, toxic gases or irradiation.
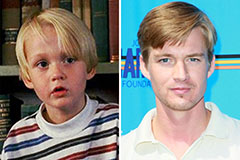 Mason Gamble Then & Now!
Mason Gamble Then & Now! Tahj Mowry Then & Now!
Tahj Mowry Then & Now! Jennifer Love Hewitt Then & Now!
Jennifer Love Hewitt Then & Now! Monica Lewinsky Then & Now!
Monica Lewinsky Then & Now! Naomi Grossman Then & Now!
Naomi Grossman Then & Now!